Please read this instruction manual carefully
safety instructions, before using the device. Keep the instruction
manual in a safe place for later reference.
If you pass the device on to a third party
must remain with the device.
GB IMPORT
Safety instructions
• This device must only be used for the purpose described in these instructions. The manufacturer
is not liable for damage resulting from improper use.
• Do not use the device in the presence of ammable anaesthetic mixtures with oxygen or nitrous
oxide (laughing gas).
• This device is only suitable for the anaesthesia and ventilation of the lungs.
• This device may only be used with the original accessories, which are listed in these instructions.
• Do not use the device, if you spot damage or you notice something unusual.
• Never open the device.
• This device consists of delicate components and must be handled with care. Observe the stor
-
age and operating conditions in the chapter “T
• Protect the device from: - water and moisture, - extreme temperatures, - knocks and drops, - dirt
and dust, - strong sunlight, - heat and cold
• Adhere to the safety regulations applicable to electrical appliances, in particular the following:
- Never touch the device with wet or moist hands. - Position the device on a level and stable sur
-
face during use. - Do not pull the power cable or the device to remove the plug from the socket.
- The power cable plug is used to disconnect the device from the power supply
always remain accessible during use.
• Before connecting the device, make sure that the electrical data on the label on the bottom of the
device match the data of the mains.
• In case the mains plug of the device does not t into the socket, contact qualied personnel to
replace the mains plug. In general, the use of adapters and extension cables is advised against. If
it is essential to use them, then they must meet the safety regulations. In this case, however
permitted limit values, which are specied on the adapters and extension cables, must always be
adhered to.
• Do not leave the device plugged in when not in use; remove the plug from the socket when the
device is not being used.
• The installation must be carried out in accordance with the manufacturer
-
rect installation can cause damage to people, animals and objects, for which the manufacturer
cannot be held liable.
• Do not replace the power cable of this device. In the case of a faulty cable, contact a technical
assistance centre approved by the manufacturer
• The power cable should always be fully unwound to avoid dangerous overheating.
• Before every cleaning or maintenance operation, the device must be switched off and the power
cable removed from the socket.
• Only use the medicine prescribed to you by your doctor and follow the instructions of your doctor
regarding dosage, duration and frequency of the therapy
• Only use the parts specied by the doctor in accordance with your specic illness.
• Only use the nose piece if expressly instructed to do so by your doctor
are NEVER inserted into the nose, but are only held as near as possible in front of the nose.
• Check on the package insert of the medicine, whether there are contraindications for use with the
usual systems for inhalation therapy
• When positioning the device, make sure that the On/Off switch can be easily reached.
• For reasons of hygiene, do not use the same accessories for more than one person.
• Do not tilt the nebuliser by more than 60°.
• Do not use the device near strong electromagnetic elds such as mobile phones or radio equip
-
ment. Keep a minimum distance of 3.3 m to such devices when using this device.
• Make sure that children do not use the device unsupervised; some parts are so small that they
could be swallowed. T
of tripping, they are not kinked and the risk of strangulation is eliminated.
• The use of this device is not a replacement for visiting the doctor
Intended use
The MEDISANA
vice is designed for the nebulisation of liquids and liquid medicines (aerosols) and for the
treatment of the upper and lower airways.
Preparing the device
Before the rst use, we recommend cleaning all components - as described in the
chapter ”Cleaning and disinfection”.
Application
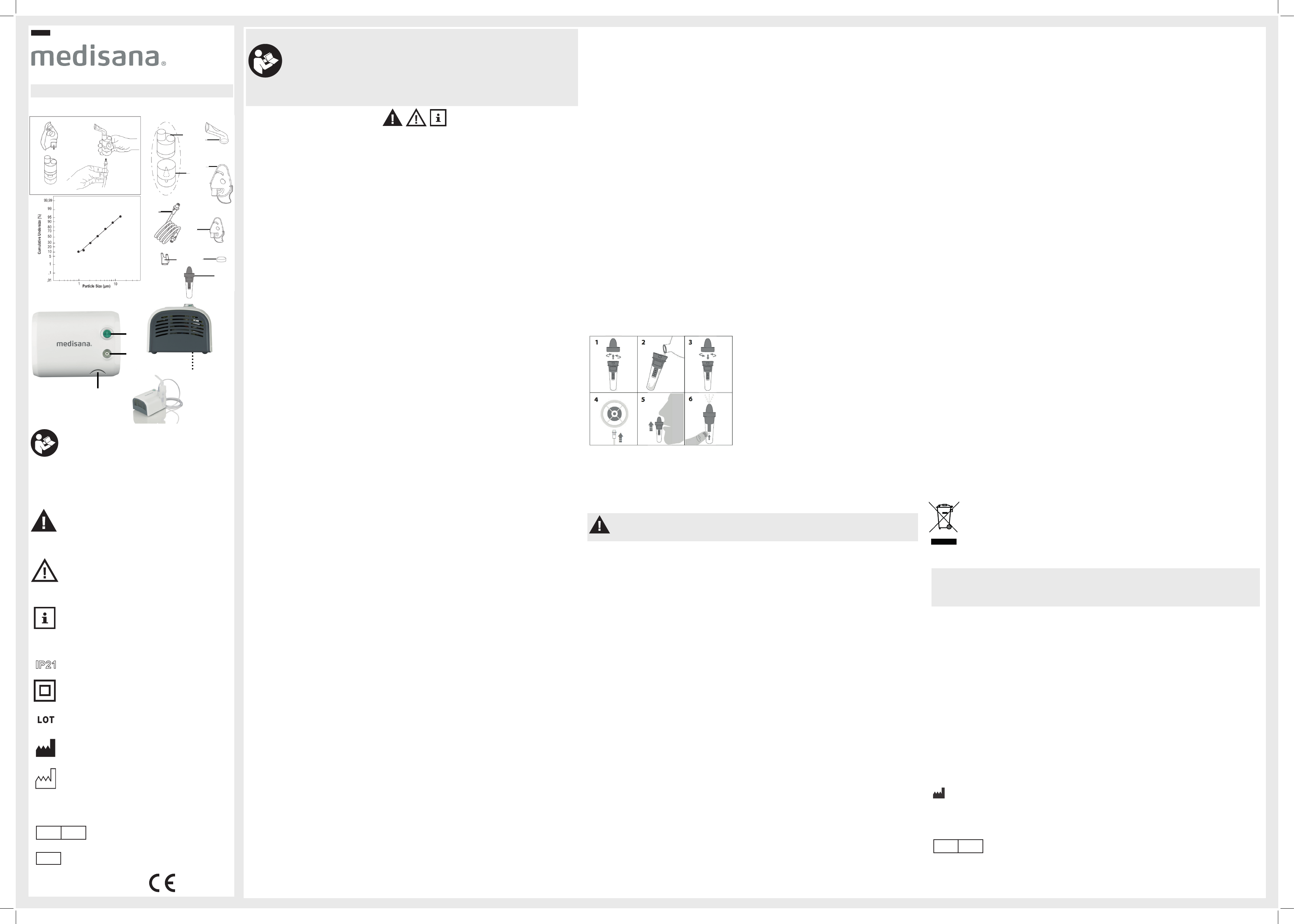
1. Assembling the nebuliser set
w
. Make sure that all parts are complete.
2. Fill the nebuliser with the inhalation solution prescribed by your doctor
exceeded.
3. Connect the nebuliser
7
via the air tube
6
to the connector
3
on the compressor and plug the power cable into
the electrical outlet (230V 50 Hz
4. T
4
to the “I” position.
- The mouthpiece guarantees better delivery of the medicine into the lungs.
- Choose between the adult
9
and child face mask
0
and make sure that the mask
fully covers the mouth and nose area.
- Use all accessories including the nose piece
5
as prescribed by your doctor
5. Whilst inhaling, sit upright and in a relaxed position at a table (not in an armchair), so as not to compress the
airways and therefore not impair the effectiveness of the treatment. Do not lie down whilst inhaling. Stop the
inhalation if you feel unwell.
6. After you
4
to the “O” position, in order to switch off the device and remove the plug from the electrical outlet.
7. Empty the remaining inhalation solution from the nebuliser and clean the device as set out in the chapter “Clean -
ing and disinfection”.
• This device was developed for operation in 30
after 30 minutes and wait a further 30 minutes, before you continue the treatment.
• The device does not require calibration. Modication of the device is not permitted.
Use of the nasal shower
e
(only IN 520)
1. Remove cover: T
2. Fill container with rinsing solution (adhere to manufacturer
3. Screw cover back on (clockwise).
4. Connect the nasal shower to the tube
6
. The other end of the tube should already be connected to the main
device at position
3
.
5. Place the nose piece onto the nostril and breath through the nose.
6. Switch the inhaler on (
4
) and guide your nger over the small opening on the lower part of the nasal shower to
begin with the treatment.
During use, you should breath in and out slowly through the nose and tilt your head slightly to the opposite side of
the affected nostril, so that the salt spray can ow deep into your nostril. Y-
ing your nger from the opening.
Scope of delivery
• 1 MEDISANA inhaler IN 510/520 (
1
position of the air lter
2
holder for the nebuliser set,
3
connector for
the air tube,
4
On/Off switch)
• 1 Instruction manual
• Accessories:
5
nose piece,
6
air tube (IN 520: two air tubes),
7
nebuliser
7
a atomiser head (
w
as-
sembling the nebuliser set),
8
mouthpiece,
9
adult face mask,
0
(IN 520: child face mask),
q
air lter
(replacement on position
1
), storage bag, (
e
IN 520: nasal shower)
Cleaning and disinfection
• Clean all accessories thoroughly after each treatment in order to remove residues of medicinal product and
possible impurities.
• Use a soft, dry cloth and a non-abrasive cleaning agent to clean the compressor
• Make sure that there is no ingress of liquids into the device and the power cable is disconnected.
Cleaning and disinfection of the accessories
Follow the instructions for cleaning and disinfecting the accessories exactly
performance of the device and the therapeutic success.
Before and after each application
1. T
7
anticlockwise in order to open the nebuliser and remove the atomiser
head
7
a.
2. W
8
, the nose piece
5
and the nasal shower
e
under running
water
3. W
4. Assemble the nebuliser parts again and connect the nebuliser to the air tube.
5. Switch the device on and leave in use for 10-15 minutes.
Use only cold sterilisation solutions in accordance with the manufacturer
Do not boil or autoclave masks and air tube.
Maintenance and care
Replacement of the nebuliser
Replace the nebuliser
7
after a relatively long period of non-use, if it has deformations or cracks or if the atom-
iser head
7
a is blocked by a dried-up medicine, dust, etc. We recommend replacing the nebuliser after 6 to 12
months depending on use. Only use the original nebuliser!
Replacement of the air lter
Under normal conditions of use, the air lter
q
should be replaced after about 500 hours of use or one year
recommend regularly checking (10-12 applications) and replacing the air lter
it feels moist. Remove the air lter (Position
1
) replace it with a new one. Do not try to clean the lter for reuse.
The air lter must not be repaired or maintained, whilst it is being used by a patient.
Only use original lter! Do not use the device without lter!
The current version of this instruction manual can be found at www
In
technical and design changes without prior notice.
W
In case of warranty please contact your specialist shop or the service centre directly
If you need to return the device, please indicate the defect and enclose a copy of the purchase receipt.
The following warranty conditions apply:
1.
The date of purchase is to be proven in case of warranty by the purchase receipt or invoice.
2. Defects due to material or manufacturing defects shall be repaired free of charge within the warranty period.
3.
4. The following are excluded from the warranty:
a.
instruction manual.
b. Damage due to repair or intervention by the purchaser or
unauthorised third parties.
c. T
or when sending it to the service centre.
d.
5. Liability for direct or indirect consequential damage caused by the device is also excluded when the damage on the device
is recognised as a warranty claim.
DE/GB
GB INSTRUCTIONS FOR USE Inhaler IN 510/520
Legend
This instruction manual belongs to this device.
The instruction manual includes important
information on the initial start-up and handling.
Read this instruction manual completely
ure to follow these instructions may result in
serious injury or damage to the device.
W
These warnings must be followed to prevent
possible injury to the user
NOTICE
These instructions must be followed to pre-
vent possible injury to the device.
PLEASE NOTE
These instructions provide you with useful
additional information regarding installation
or operation.
Information about protection type against for-
eign objects and water
Protection class II
LOT number
Manufacturer
Date of manufacture
Off/on
Authorised EU representative
Serial number of the device
Device and controls
Malfunctions and countermeasures
The device cannot be switched on
• Make sure that the power cable is correctly plugged into the electrical output.
• Make sure that the On/Off switch
4
is in the “I” position.
• Make sure that the device has been operated within the operating period specied in
these instructions (30 min. on / 30 min. off).
The device is only misting a little or not at all
• Make sure that the air tube
6
is properly attached on both ends.
• Make sure that the air tube
6
is not compressed, bent, dirty or blocked. If necessary
replace it with a new one.
• Make sure that the nebuliser
7
is completely assembled and the coloured atomiser head
7
a has been correctly positioned and is not blocked.
• Make sure that the required inhalation solution has been poured in.
2
54547/54548 09/2019 V
0123
IP21
Globalcare Medical T
7th Building, 39 Middle Industrial Main Road,
European Industrial Zone, Xiaolan T
528415 Zhongshan City
PEOPLE‘S REPUBLIC OF CHINA
imported & distributed by
MEDISANA
Jagenbergstraße 19
41468 NEUSS
GERMANY
Donawa, Lifescience Consulting Srl
Piazza Albania,
00153 Rome / Italy
EC REP
EC REP
SN
1
W
Make sure that children do not get hold of the packaging lms.
There is a risk of suffocation!
Name
Power supply
Nebulisation amount (average)
Particle size
max. pressure
Noise level
Nebuliser ll quantity
Residual amount
Operating period
Expected service life
Operating conditions
Storage and transport conditions
Weight
Dimensions
Length of the power cable
IP class
Reference to standards
Item number
EAN number:
MEDISANAinhaler IN 510/520
230 V~ 50 Hz
0.35 ml/min.
3.07 μm
2.3 bar
52 dBA, 1 m
min. 2 ml; max. 8 ml
0.8 ml
30 min. on / 30 min. off
1000 hours
10 - 40 °C / 50 - 104 °F
30 - 85 % relative maximum humidity
700 - 1060 hPa air pressure
-25 - +70 °C / -13 - +158 °F
10 - 95 % relative maximum humidity
700 - 1060 hPa air pressure
1450 g
18 x 14 x 9.4 cm
180 cm
IP 21
EN 13544-1; EN 60601-1; EN 60601-2; EN 60601-1-6;
IEC 60601-1-1
54547/54548
40 15588 54547 4/40 15588 54548 1
This device must not be disposed of with domestic waste.
All users are obliged to bring all electrical or electronic devices to a collection point in their
town/city or to a retailer
that they can be disposed of in an environmentally responsible manner
Please contact your local authority or retailer with regard to disposal procedures.
T
O/I
4
3
7
8
9
7
a
6
0
5
q
w
This device meets the requirements of the Directive concerning medical devices 93/42/EEC.
Device in Class II in relation to protection against electric shocks. Nebuliser
are applied parts of type BF
e
Empty the remaining rinsing solution from the nasal
shower after the treatment and clean the device as set
out in the chapter “Cleaning and disinfection”.