11
Zulassungsbestimmungen
und Sicherheitshinweise
Modellbezeichnung: FB502
The scale measures body fat using bioimpedance analysis. ITO electrodes form a pattern
on the top of scale, and send a small, safe signal through the body to measure impedance.
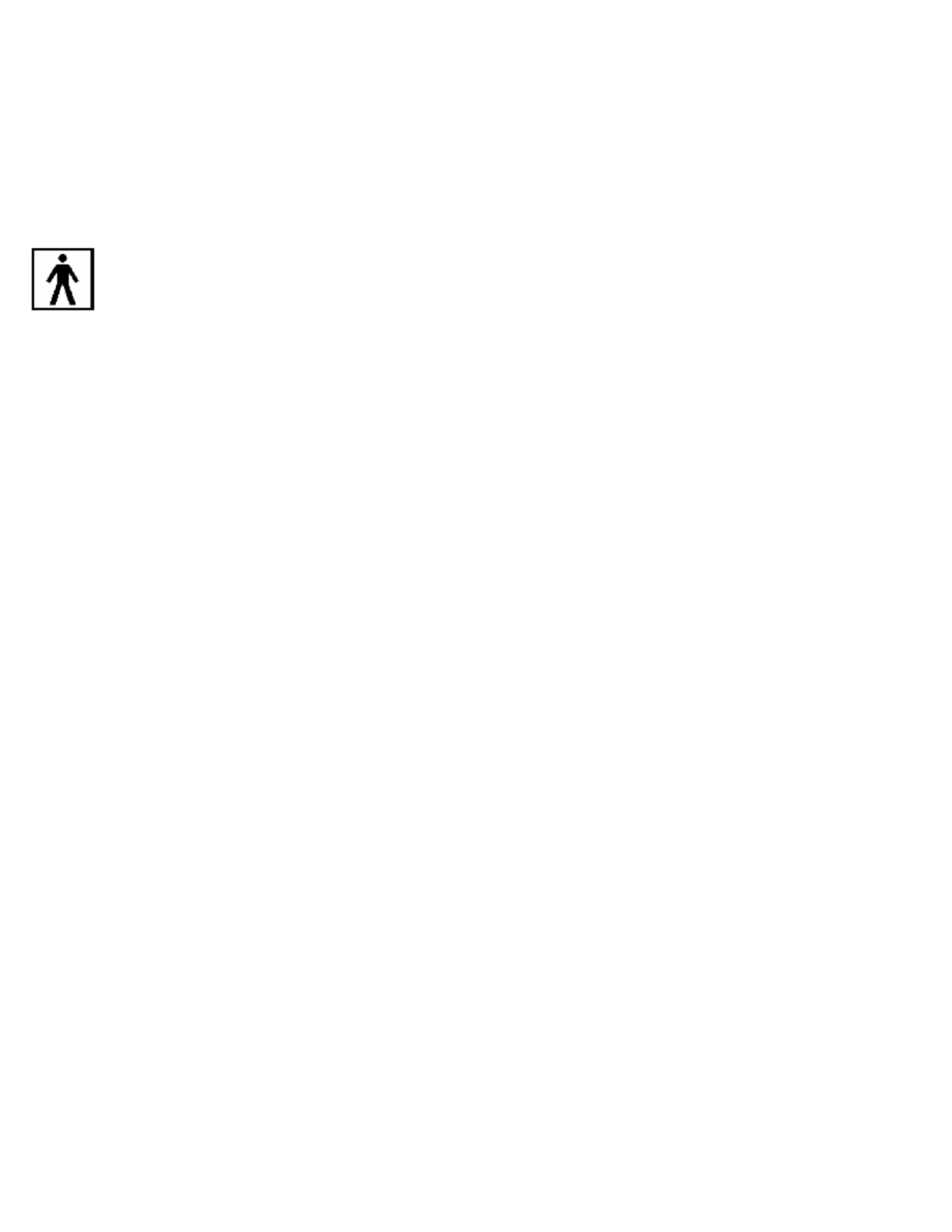
Type BF Applied Part
This symbol indicates that the surface scale is a Type BF applied part intended to deliver
an electrophysiological signal to and from the user. The scale measures body mass using
bioimpedance analysis. ITO electrodes form a pattern on the top of scale, and send a
small, safe signal through the body to measure impedance.
Aria 2 is not considered a medical device in all countries below except for the U.S.
USA: Erklärung der Federal Communications
Commission (FCC)
This device complies with Part 15 of the FCC Rules.
Operation is subject to the following two conditions:
This device may not cause harmful interference and
This device must accept any interference received, including interference that may cause
undesired operation
FCC Warning
Changes or modifications not approved by Fitbit, Inc. could void the user’s authority to
operate the equipment.
Note: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following
measures:
Reorient or relocate the receiving antenna
Increase the separation between the equipment and receiver